
Ingredients : Promega launches XpressAmp Direct Amplification reagents. Packaging : Neopac wins Tube Council award for pharma packaging. Research : Almac Group announces PhD scholarship at UCL. Equipment : Delegate tickets now on sale for Manufacturing Chemist Live. Contract Manufacturing : Fujifilm Diosynth commences cell culture expansion project.
In a low-temperature liquid chemical sterile processing system, several steps must be followed for effective sterilization: 1. Pre-cleaning of the devices is necessary because many devices have small connected lumens. 2. Leak testing is done to ensure there are

A growing demand for sterile filter from the pharmaceutical sector is one of the significant factors influencing the market growth. Market Size – USD 4,670.1 Million in 2019, Market Growth - CAGR of 7.7%, Market Trends – Rising usage of disposables

There are several types of mechanical filters are available for this method such as Chamberland Filters, Berkefeld Filters, Mandler Filters, Fritted-Glass Filters, Asbestos Filters, Jenkins Filter, Ultrafilter, Membrane Filter, Cleaning Filters Radiation Ultraviolet light

Your one-stop solution for small quantity containment and delivery. By offering a limited number of ready-to-use products in small packs, we can provide you with easy ordering and fast delivery for your small-quantity stopper, seal, vial and syringe needs. Check back often for additional product offerings!

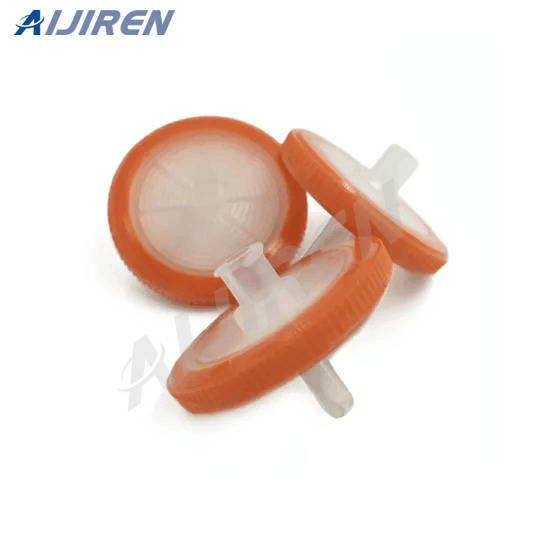
Syringe Filter Nylon PTFE PTFE Venting Filters PVDF MCE CA GF Sterile Reusable G-MP Disc Membrane Filter OEM SimplePak Blotting PES MCE Nylon PVDF PTFE PP CA Nylon Mesh PC QC Vacuum Filter Disposable Glass Solvent Multi Vacuum Filter Vacuum Pump

8040582 Filter Block Asby Far RED Cytation 5, Cytation Multi-Mode, Synergy H1 8040583 Green/LUM Filter Cube - Green Filter Set: EX 485/20 | EM 528/10 | DM 510 Cytation 1, Cytation 5, Synergy H1 8040584 Filter Cube: Blue (360/460) / Red (530/590) , ,

PharmaManufacturing.com is the site for knowledge, news and analysis for manufacturing and other professionals working in the pharmaceutical, biopharmaceutical and biotech industries. It’s no secret that the pipeline for Alzheimer’s treatments has mostly become

PUR Water Pitcher Replacement Filter with Lead Reduction, 3 Pack, Blue. 4.6 out of 5 stars. 12,894. $24.88. $24. . 88 ($8.29/Count) $23.64 with Subscribe & Save discount. This three-pack is compatible with all PUR water pitchers, regardless of size.

Label the syringe with both patient and blood product identification details including expiry date and time of blood product. If rapid transfusion of small volumes is required, draw the required volume into a syringe through a 170 to 200 micron filter. Burettes should not be used for transfusion of blood products.

Keep up with advances in Visual Inspection technology! There is still time to register for the Visual Inspection Forum, with a special focus this year on automated technology, artificial intelligence applications, and regulatory requirements governing the VI process.

Sterile syringe access is an important means to reduce HIV risk, but many injection drug users (IDU) who obtain syringes from sterile sources continue to share syringes.

2021/1/1 · China Colombia Costa Rica Croatia Cyprus Czech Republic Denmark Dominican Republic Ecuador Egypt El Salvador Estonia Finland France Georgia Germany Greece Guatemala Hong Kong Hungary Iceland India Indonesia Iraq Ireland (Republic Of) Israel Italy

4.13.4.2 Using Filter Membranes for Increased Diffusion Filter membranes are also used as culture substrates to support the long-term viability of tissue slices and layers of cultured cells. Unlike nonporous culture surfaces, culture medium can diffuse through porous filter membranes and cellular waste products can diffuse away from the cell mass into the membrane.

2020/11/25 · Medications that are required to be sterile include those administered through injection, intravenous infusion (IV), intraocular (injection in the eye) or intrathecal (injection in the spine). Understanding the risks inherent in sterile compounding and incorporating established standards are essential for patient safety.